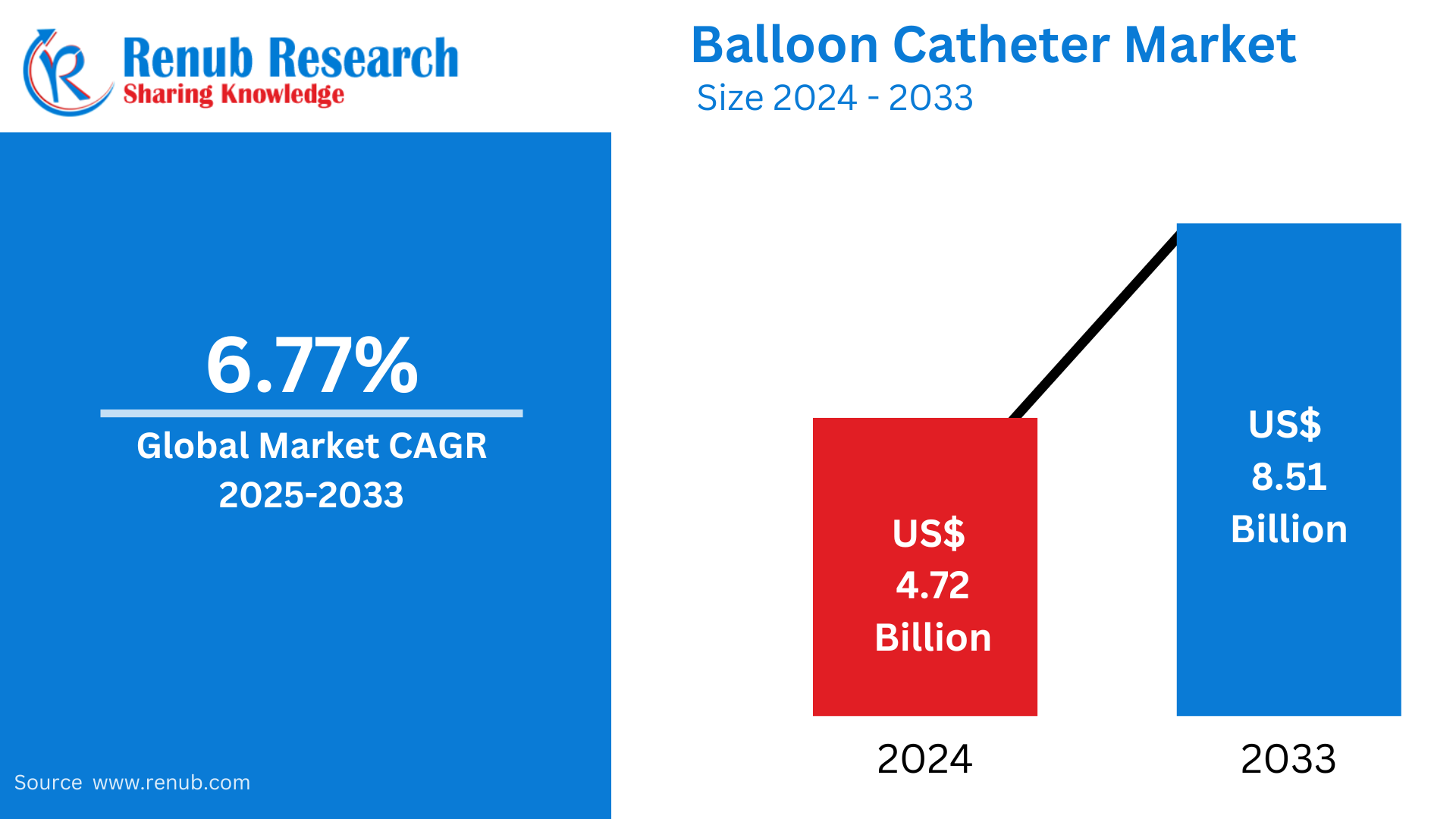
Global Balloon Catheter Market Size and Forecast 2025–2033
According to Renub Research global balloon catheter market is set for solid expansion over the next decade, increasing from US$ 4.72 billion in 2024 to US$ 8.51 billion by 2033, registering a CAGR of 6.77% between 2025 and 2033. Growth is primarily driven by rising incidences of cardiovascular and peripheral vascular diseases, increasing preference for minimally invasive procedures, continuous advancements in catheter design and materials, and expanding aging populations worldwide. With healthcare systems emphasizing faster recovery, improved outcomes, and reduced hospital stays, balloon catheters continue to be essential devices in modern interventional medicine.
Balloon Catheter Market Overview
A balloon catheter is a flexible medical device with an inflatable balloon at its tip, used predominantly in angioplasty and related minimally invasive procedures to open narrowed or blocked blood vessels. By inflating within the vessel, the balloon restores normal blood flow, significantly reducing heart-related complications. Beyond cardiology, balloon catheters are widely used in peripheral vascular interventions, as well as non-vascular procedures involving dilation of the urinary tract, gastrointestinal tract, and esophagus. Drug-coated balloon catheters enhance therapeutic effectiveness by enabling localized drug delivery, reducing restenosis risks. Their precision, versatility, and minimally invasive approach make balloon catheters indispensable tools in both diagnostic and therapeutic applications.
Download Free Sample Report:https://www.renub.com/request-sample-page.php?gturl=balloon-catheter-market-p.php
Key Growth Drivers in the Balloon Catheter Market
Increasing Prevalence of Cardiovascular Diseases
Global cardiovascular diseases remain among the leading causes of mortality, significantly boosting balloon catheter demand. Conditions such as coronary artery disease (CAD) and peripheral artery disease (PAD) frequently require angioplasty procedures. Lifestyle-related risk factors, aging populations, and improved diagnostics are increasing patient volumes. For instance, national health statistics frequently indicate rising heart and vascular disease prevalence, underscoring the continuous need for reliable interventional devices. Growing awareness, early treatment initiatives, and expanding healthcare infrastructure across both developed and emerging economies are expected to sustain strong catheter adoption.
Technological Advancements Enhancing Efficiency
Continuous improvements in material engineering and catheter design are transforming clinical outcomes. Innovations like drug-eluting and drug-coated balloon catheters, improved imaging compatibility, enhanced flexibility, reduced profile sizes, and improved pushability ensure safer and more precise interventions. These advancements reduce restenosis risks, increase procedural success rates, and expand applicability in complex vascular cases. The introduction of specialized catheters and new CE-marked and FDA-cleared products supports consistent technological evolution across global markets.
Rising Adoption of Minimally Invasive Procedures
Minimally invasive surgery trends are reshaping global healthcare practice, with patients and physicians favoring shorter recovery times, minimal scarring, and fewer complications. Balloon catheters are fundamental to endovascular interventions and angioplasty procedures, making them central beneficiaries of this shift. Enhanced visualization technologies and advanced catheter navigation systems are further strengthening the effectiveness and accessibility of minimally invasive treatments worldwide.
Key Market Challenges
High Cost and Limited Accessibility
High costs associated with advanced balloon catheter types—such as drug-coated or cutting balloons—pose challenges to widespread adoption, particularly in low- and middle-income countries. Additionally, the need for trained specialists and advanced imaging infrastructure increases overall treatment expenses. Healthcare disparities between urban and rural regions also affect accessibility. Enhancing cost efficiency, developing price-sensitive products, and improving healthcare infrastructure are essential to overcoming these barriers.
Stringent Regulatory Landscape
Balloon catheter manufacturers must comply with rigorous global regulatory frameworks, including extensive clinical testing and regional approval requirements. Evolving regulatory standards, especially in regions governed by EU MDR and stringent U.S. FDA approvals, may prolong product launches and increase development costs. These complexities sometimes deter smaller companies and slow innovation. Collaborative regulatory strategies and streamlined approval pathways are crucial to maintaining innovation momentum.
Country-Level Market Insights
United States
The U.S. remains a dominant market, supported by high cardiovascular disease prevalence, strong healthcare infrastructure, widespread acceptance of minimally invasive procedures, and high R&D investments. Innovations such as drug-coated balloon catheters and advanced guided systems are widely utilized. However, high procedural costs and strict regulatory standards present continuing challenges.
Germany
Germany leads within Europe due to advanced healthcare systems, technological innovation, and increasing cardiovascular cases among its aging population. Strong reimbursement policies and collaboration between healthcare institutes and manufacturers further strengthen market expansion. Despite stringent MDR compliance requirements, Germany remains a central hub for adopting next-generation cardiac devices.
China
China represents one of the fastest-growing balloon catheter markets globally, driven by expanding healthcare infrastructure, increasing cardiovascular case loads, and government initiatives supporting domestic medical device manufacturing. While disparities between rural and urban healthcare accessibility persist, technological advancements and local production are helping bridge the gap, positioning China as a major future contributor to global market value.
Brazil
Brazil leads Latin America’s balloon catheter landscape with upgraded healthcare infrastructure, rising awareness of cardiovascular healthcare, and growing adoption of minimally invasive treatments. Public and private healthcare initiatives support greater access to angioplasty and related interventions. Although economic constraints remain, market prospects continue to strengthen.
Saudi Arabia
Saudi Arabia is emerging as a strong regional market in the Middle East due to healthcare modernization under Vision 2030, rising cardiovascular disease rates, expanding hospital infrastructure, and growing adoption of innovative medical devices. Strategic partnerships and increased regulatory support further strengthen market potential.
Market Segmentation Overview
By Product Type: Normal | Drug-Eluting | Cutting | Scoring | Stent Graft | Others
By Indication: Coronary Artery Disease | Peripheral Vascular Disease
By Raw Materials: Polyurethane | Nylon | Others
By End User: Hospitals | Clinics | Ambulatory Surgical Centers | Diagnostic Centers
Regional Coverage
North America (United States, Canada) | Europe (France, Germany, Italy, Spain, United Kingdom, Belgium, Netherlands, Turkey) | Asia-Pacific (China, Japan, India, South Korea, Thailand, Malaysia, Indonesia, Australia, New Zealand) | Latin America (Brazil, Mexico, Argentina) | Middle East & Africa (Saudi Arabia, UAE, South Africa) | Rest of the World.
Competitive Landscape
The balloon catheter market is moderately competitive, characterized by innovation-driven strategies, product enhancements, regulatory approvals, mergers, and global expansion. Leading companies include:
Abbott Laboratories, Becton Dickinson and Company, Cardinal Health, Teleflex Incorporated, Medtronic Plc., Johnson & Johnson, Edwards Lifesciences Corporation, Stryker Corporation, and Smith & Nephew. These players focus on technological innovation, enhanced safety features, clinical research, and strategic partnerships to strengthen global market positioning.
Conclusion – Strong Growth Outlook Ahead
With rising cardiovascular disease prevalence, greater reliance on minimally invasive procedures, continuous technological advancement, and expanding global healthcare capabilities, the balloon catheter market is expected to experience sustained growth through 2033. Despite regulatory complexities and cost barriers, increasing innovation, improving accessibility, and enhanced patient outcomes will continue driving adoption, firmly establishing balloon catheters as essential tools in modern interventional healthcare worldwide.